Clinical Trial - Generic IMP creation
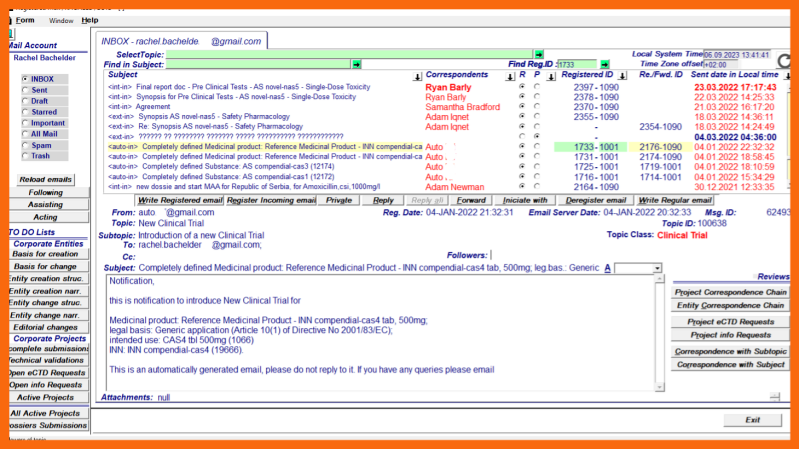
The creation of a new Clinical Trial - Generic Investigational MP begins with a "basis for creation" regular/enriched email message/notification with the topic "New Clinical Trial":
- enriched email notification sent when a Generic Active Substance, intended for generic application (Article 10(1), with Reference Medicinal Product not administered directly into the blood stream, is completely structurally defined
- enriched email notification sent when an Active Substance, intended for hybrid application (Article 10(3), is completely structurally defined
- enriched email notification sent, if the Generic Active Substance has already been introduced into the Integral Information System, and its new Reference Medicinal Product, intended for generic application (Article 10(1), which is not administered directly into the bloodstream is completely structurally defined
- enriched email notification sent if the Generic Active Substance has already been introduced into the Integral Information System and its new Reference Medicinal Product, intended for hybrid application (Article 10(3), is completely structurally defined
- regular/enriched email message sent when there is a need for additional Clinical Trial with Generic Investigational Medicinal Products

Gathering of the documentation/data
Gathering of documentation and necessary data for the structural and narrative definition of a new Clinical Trial - Generic Investigational MP can be done in advance, before the beginning of structural definition and subsequently when the Clinical Trial - Generic Investigational MP has become a topic in the enriched email correspondence.

Before the beginning of the structural definition
Gathering of documentation related to the creation of a new Clinical Trial - Generic Investigational MP a process that can begin before the receipt of the "basis for creation" enriched email and subsequent beginning of the structural definition. During that period database record for the new Clinical Trial - Generic Investigational MP and directory tree of narrative replacement files does not exists in the eCTD Integrated Information System and the only topic that can be used in the correspondence is "New Clinical Trial". Attached documents are classified and uploaded to the Corporate Documentation Cloud but they are not linked with the Clinical Trial - Generic Investigational MP that is being created. Nevertheless, linking of all correspondence to the newly created Clinical Trial - Generic Investigational MP is essential and it can be done when the structural definition is started.

After the beginning of the structural definition
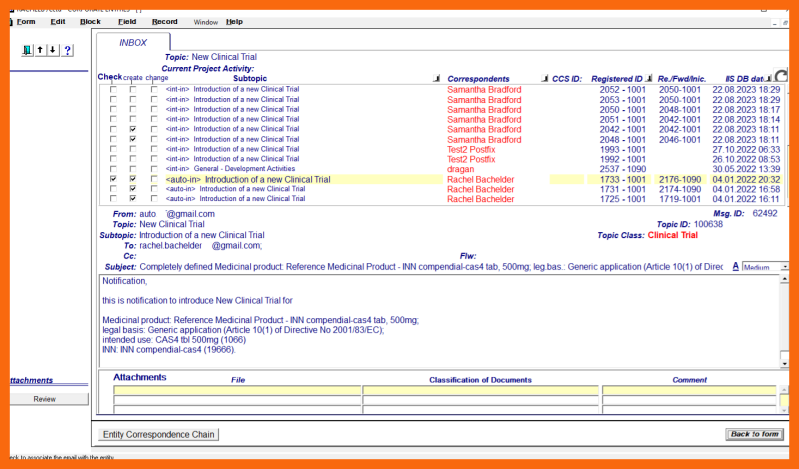
The clinical entity person begins the process of Clinical Trial structural definition right after receiving the "basis for creation" enriched email. When selecting a "basis for creation" enriched email on the "correspondence" sub-application form, all related correspondence previously received relating to the Clinical Trial being created should also be selected.
During the insertion of the record into the eCTD Integrated Information System a company core sequence and a directory tree of replacement narrative files are generated and the newly defined Clinical Trial automatically becomes the topic of the "basis for creation" enriched email and all selected previously received enriched emails. That can be seen when inserting additional data on the "correspondence" sub-application form.
Previously received attachments/documents have to be manually assigned to the corresponding replacement files using the application form "Corporate Entity creation / Clinical Trial / Narrative data". The process is explained in the narrative definition section.
All correspondence from then on should be conducted using enriched email messages and the newly-introduced Clinical Trial as the topic.
During the subsequent correspondence and classification of the attachment(s), attached document(s) are automatically uploaded to the Corporate Documentation Cloud and assigned to the appropriate replacement files.

Structural definition
In order to create and structurally define a Clinical Trial following business processes must be performed in the presented order:
- Generation/receipt of a "basis for creation" regular/enriched email
- Selection of one "basis for creation" enriched email message/notification and related correspondence on the sub-application form "Correspondence" of the application form "Corporate Entity creation/Clinical Trial/Structural Data"
- Definition of general structural data of the Clinical Trial
- Definition of Clinical Trial Objectives and selection of Investigational Sites
- Definition of Clinical Trial Design and Investigational Medicinal Product(s)
- Definition of Subjects Groups
- Completion of the structural definition
- Complete structural definition during the first instance/session
- Complete structural definition in several instances/sessions

1. Generation/receipt of a "basis for creation" regular/enriched email

The enriched email notification automatically sent to the high-level clinical entity person after the creation and complete structural definition of the Active Substance with the intended legal basis for marketing authorization in accordance with Article 8(3).
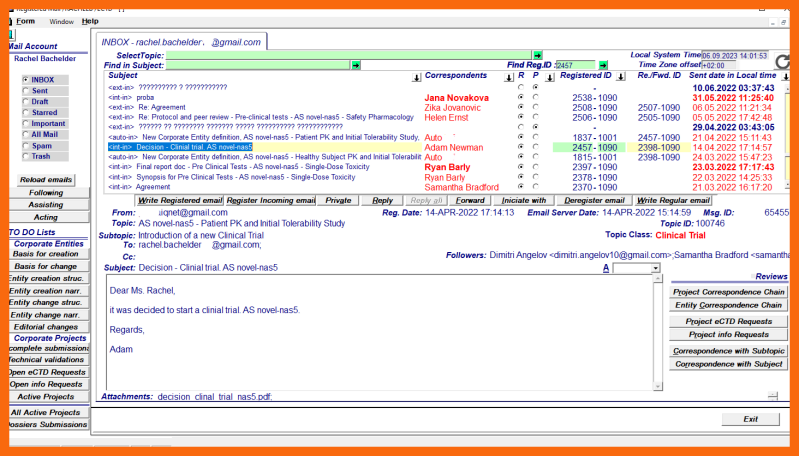
The regular/enriched email message with the attachment "Decision to introduce a new Clinical Trial" sent by a corporate person with the appropriate authority to the high-level clinical entity person when there is a need for an additional Clinical Trial.
The regular email message must be registered by the recipient.
If necessary, additional correspondence using the same topic and subtopic can be made in order to clarify certain details.
When all details are clarified, the structural definition process can be started by choosing the menu item "Corporate Entity creation/Clinical Trial/Structural Data" on the "Corporate Entities" application module.

2. Selection of one "basis for creation" enriched email message/notification

To create a new Clinical Trial, first, using the "Correspondence" sub-application form, a received "basis for creation" enriched email relevant to the new Clinical Trial being created must be selected from the non-processed correspondence documents. In addition, all enriched email messages classified as "related correspondence" pertaining to the same new Clinical Trial should also be selected in order to properly establish entity creation correspondence environment.

3. Definition of general structural data of the Clinical Trial

A minimal set of structural data is specified with the (*) and they are a prerequisite for the insertion of the record in the Integrated Information System Database.
The Clinical Trial's structural data is defined by using:
- Internal title - automatically generated
- Active Substance - transferred from the "basis for creation" enriched notification/message
- Reference Medicinal Product - transferred from the "basis for creation" enriched notification/message
- Intended legal basis - transferred from the "basis for creation" enriched notification/message
- Sponsor(s) - selected form the LoV of corporate Organizations
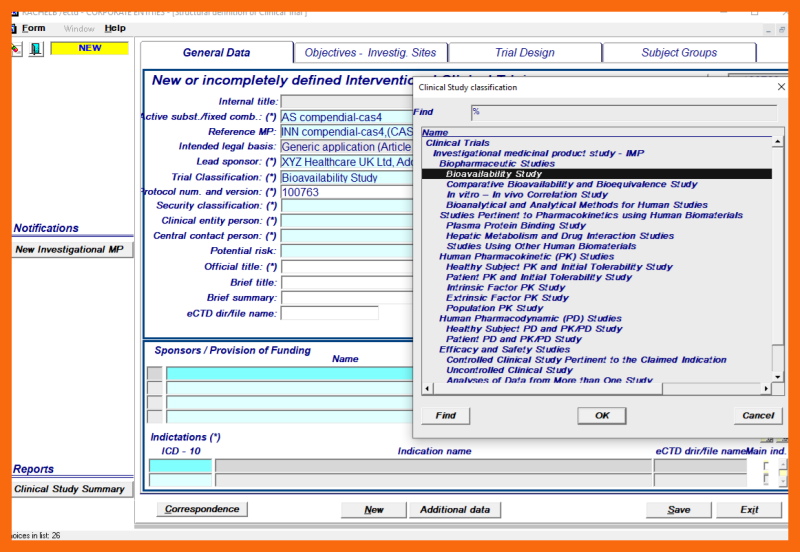
- Trial classification - selected from the LoV
- Protocol (number and version) - free text field
- Security classification - selected from the LoV
- public
- internal
- confidential
- restricted
- Designated clinical entity person - selected form the LoV of persons with corresponding security clearance
- Central contact person - selected form the LoV of persons with corresponding security clearance
- Potential risk - selected from the LoV
- Official title - free text field
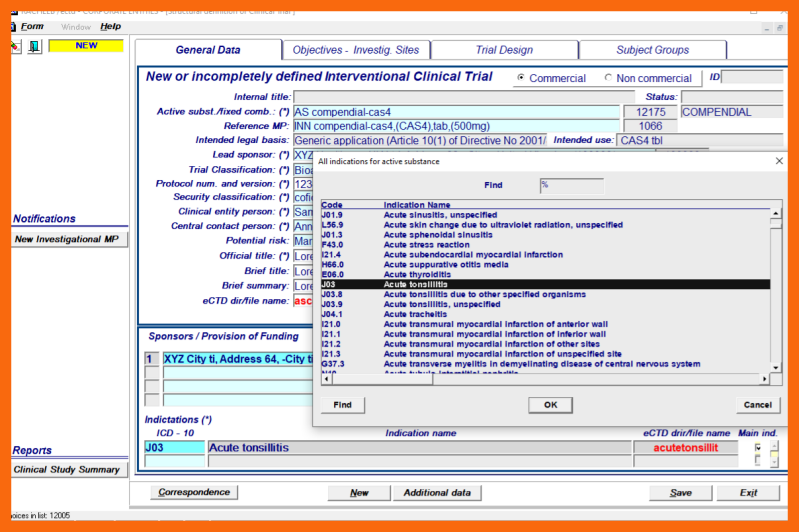
- Indication(s) - selected from the LoV in accordance with ICD - 10 classification

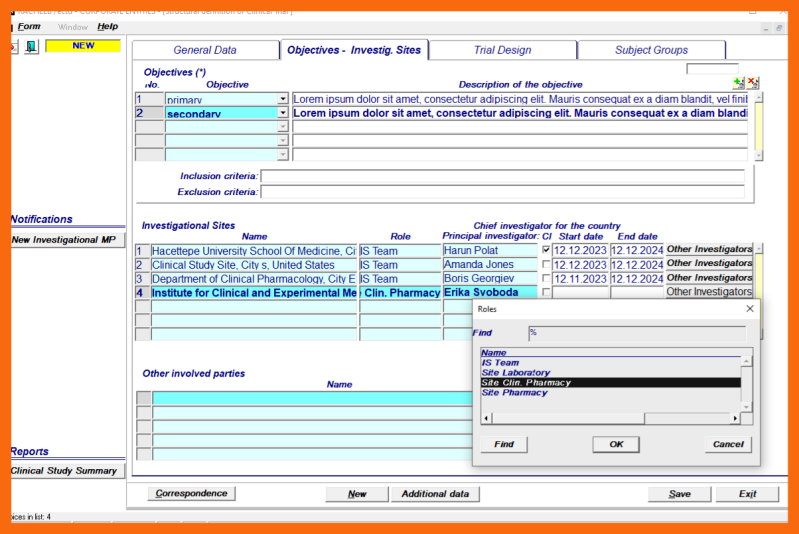
4. Definition of Clinical Trial Objectives and selection of Investigational Sites

Objectives
In the free text field several objectives can be defined of three types:
- primary
- secondary
- safety
Several Investigational Sites can be selected from the LoV of Organizations of the appropriate type.
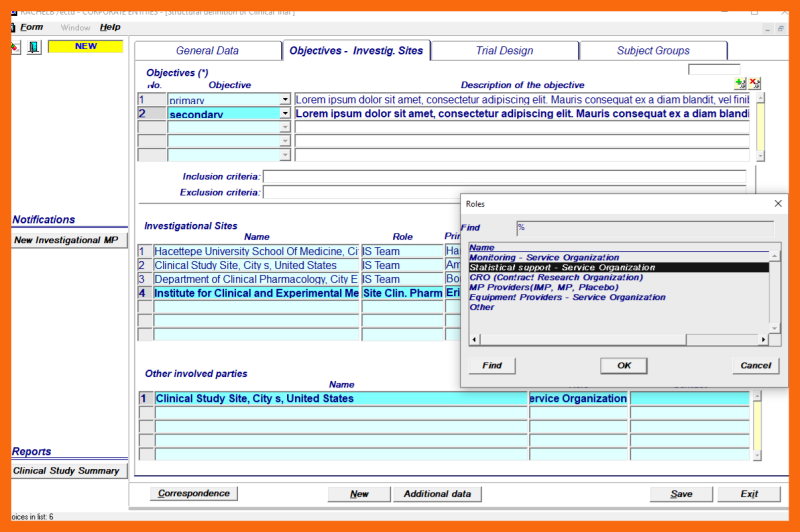
Other involved parties can be selected from the LoV of Organizations for the following roles:
- Monitoring
- Statistical support
- Contract Research Organization
- Medicinal Product providers
- Equipment providers
- Other

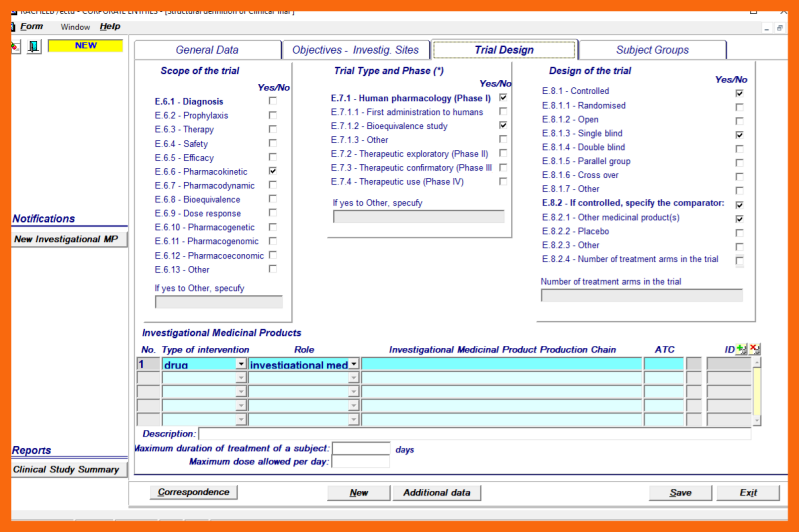
5. Definition of Clinical Trial Design and Investigational Medicinal Product(s)

Clinical Trial Design
The Clinical Trial Design can be defined by using selection blocs to specify:
- Scope of the trial
- Trial type and phase
- Design of the trial
- Species of animals
Free text fields are provided for:
- Maximum duration of treatment of a subject
- Maximum dose allowed per day
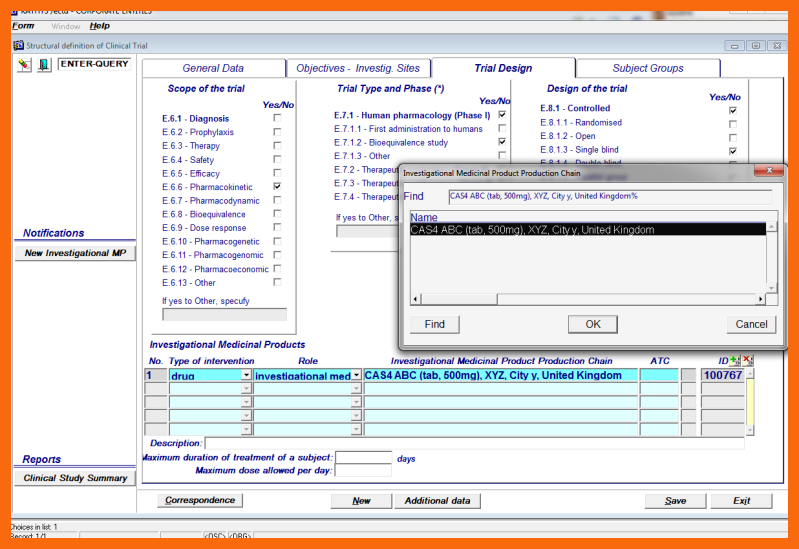
Investigational Medicinal Preparation Production Chain(s)
Several Investigational Medicinal Preparation Production Chains can be selected from the LoV of Investigational Medicinal Preparation Production Chains for the Active Substance of the Clinical Trial.

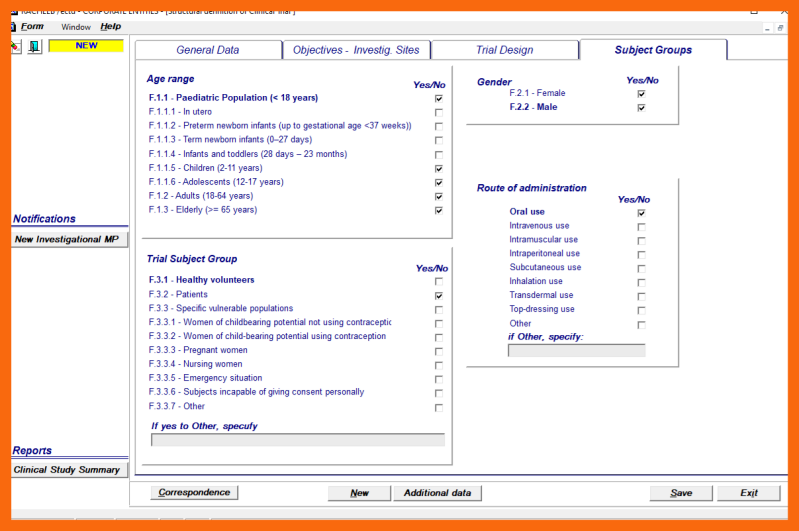
6. Definition of Subjects Groups

The Clinical Trial Subject Groups can be defined by using selection blocs to specify:
- Age range
- Trial Subject Groups
- Gender
- Route of administration
When the minimal structural data set criterion is met and the Clinical Trial entity is introduced into the Integrated Information System a core sequence, directory tree of replacement narrative files, and a registered email notification to the designated clinical entity person are generated.
At the same time, the Clinical Trial Project is initiated by the automatic compilation of the initial submission, and the clinical project person is notified with an automatically generated enriched email notification to prepare and upload the initial submission project documents.
The files that will be generated at the end of the Clinical Trial are marked with an "F" and do not prevent the initial submission from being submitted to the Investigational Sites.
During subsequent correspondence and classification of attachment, the attached document(s) are automatically uploaded to the Corporate Documentation Cloud and assigned to the appropriate replacement narrative file(s).

7. Completion of the structural definition

The structural definition process can be completed in two ways:
- In one session when all structural data are defined
- in several sessions where
- in the first session only minimal data set and maybe some of the additional data are defined
- in the next session(s) where remaining additional data are defined
Upon completion of the structural definition process, the Clinical Trial is assigned the status "completely structurally defined".
At the same time:
- an automatically generated "basis for creation" enriched email notification(s) is sent to the top-level clinical entity person with the information that the Clinical Trial Dossier(s)/Dossier extension(s) is created and the Clinical Trial Authorization Project(s) launched
- an automatically generated enriched email notification(s) is sent to the clinical project person(s) with the information that a Clinical Trial Authorization Project is launched in their domain of responsibility

7.1 Complete structural definition during the first session

The minimal data set denoted with (*) is prerequisite for the insertion of the record into the eCTD Integrated information System.
If the minimal data set requirement is met, then confirmation of the complete structural definition is prompted. If the complete structural definition is confirmed, the record is inserted into the database of the eCTD Integrated Information System with the status "completely structurally defined".

7.2 Complete structural definition in several sessions

In the first session only the minimal data set and maybe some additional data are defined. At the end of the session, confirmation of the complete structural definition is prompted. If the answer is negative, the record is inserted and the status of the entity is set to "incompletely structurally defined".
At the insert of the record, a core sequence, directory tree of replacement narrative files, and the enriched email notification to the designated clinical entity person are generated.
Additional data can be added on the same application form by clicking on the "Additional data" button and selecting the required Clinical Trial from the LoV of incompletely defined Clinical Trials. Previous enriched emails related to the selected Clinical Trial can be seen on the "correspondence" sub-application form.
The structural definition is completed when a positive response is given to the prompt for a complete structural definition. The Clinical Trial is assigned the status "completely structurally defined" with the ability to be used to define corporate entities of the higher level.

Narrative definition
The designated clinical entity person is responsible for the complete narrative definition of the Clinical Trial.
The eCTD documents can be either:
- created by the designated clinical entity person and manually uploaded to the Corporate Documentation Cloud or
- collected from related Business Partners via email correspondence and automatically uploaded to the Corporate Documentation Cloud during the enriched email processing of the Business Partner's regular email messages.
Therefore, in order to narratively define a Clinical Trial following business processes must be performed:
- Creation of eCTD documents and manual uploading to the Corporate Documentation Cloud
and/or - Collection of eCTD documents via email correspondence and automatic uploading to the Corporate Documentation Cloud
- Completion of the process

1. Creation of eCTD documents and manual uploading to the Corporate Documentation Cloud

Using any regular text processor eCTD documents have to be created and pdf files generated for uploading to the Corporate Documentation Cloud. If required, the process of verification by a senior manager can be included upon request.
The process is initiated by choosing the menu item "Corporate Entity creation / Clinical Trial / Narrative Data" on the "Corporate Entities" application module.
Other than that, the process is same as described in the "Substance - creation" page of this site, here.

2. Collection of eCTD documents via email and automatic uploading to the Corporate Documentation Cloud

In case the eCTD documents were originally generated by the Business Partner, they can be obtained via email correspondence.
The process is same as described in the "Substance - creation" page of this site, here.

3. Completion of the process

When all the presented replacement files in the directory tree are replaced with written eCTD files, the process ends automatically and the Clinical Trial entity is set to be "completely structurally and narratively defined".