Changing Corporate Entities
Medicinal Product
Changes to the Medicinal Product entity begin with structural changes on the application form "Corporate Entity change/Medicinal Product /structural data" based one or more "basis for change" documents generated within the Pharmaceutical Corporation itself or by Business Partners and processed by the Enriched Email Client.
The required "basis for change" documents are legislatively defined in:
"Guidelines on the details of the various categories of variations..." EUR-Lex Document 52013XC0802(04)
The "basis for change" document(s) may originate from the corporation itself and be delivered via:
- Enriched email message as an attached and appropriately classified document(s) or,
if originating from other Business Partners, via: - Regular email message as an attached document(s). The first recipient in the corporation is responsible for the correct processing and attachment classification of such regular email message using the Enriched Email Client
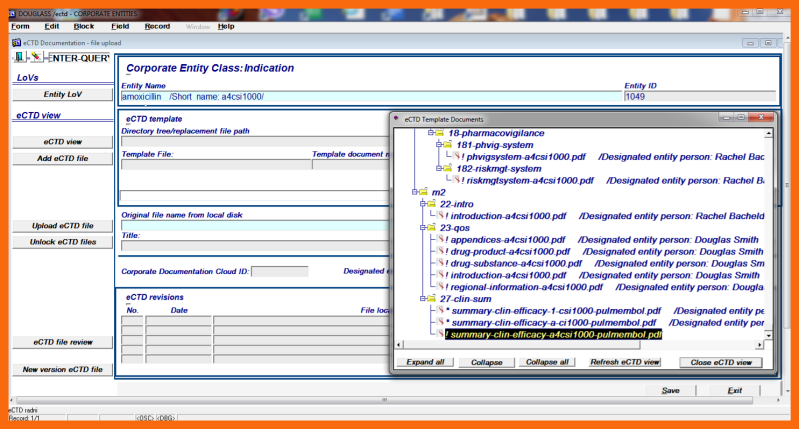
Each attachment processed with the Enriched Email Client and uploaded to the Corporate Documentation Cloud has a unique ID number. In the eCTD Integrated Information System, two additional attributes are also saved, the original file name and the assigned file name. The assigned file names are automatically created taking into account the allowable path length of the windows operating system, each Medicinal Product unique "var" synonym, and the role of the file in the eCTD context.
Since each Medicinal Product has a cmc entity person as a “designated follower”, copies of all enriched email messages/notifications related to the Medicinal Product are automatically sent to that person.
The "basis for change" documents
The following classes of "basis for change" documents are appropriate as a basis for change for a Medicinal Product entity:
- Classes of documents related to the Medicinal Product general characteristics
- WHO - ATC code acceptance as per A6#1 req.
the automatically assigned file name "va6-who-atc-code-acceptance-medprod.pdf"
- WHO - ATC code acceptance as per A6#1 req.
- Classes of documents related to the MP Safety, Efficacy and Pharmacovigilance Changes
Variations
Based on the legislatively defined set of some of the above specified "basis for change" documents, changes in the definition of Medicinal Product initiate the following Variations that have to be reported to the competent Health Authorities:
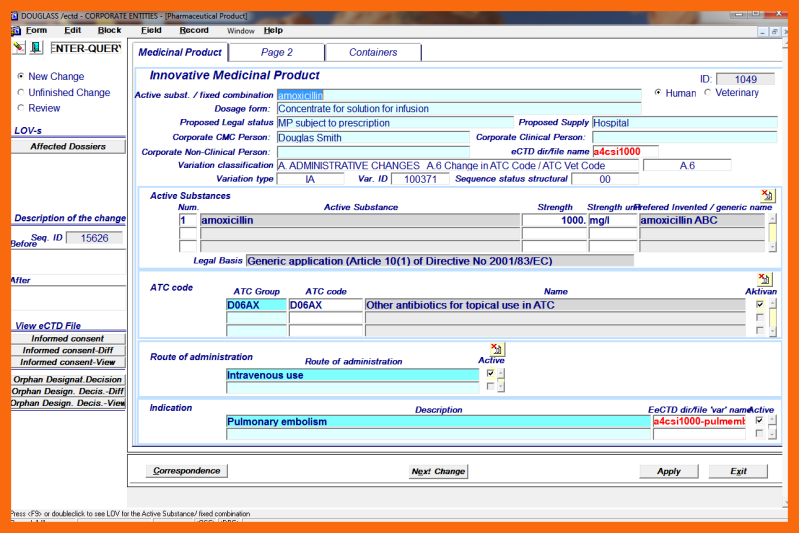
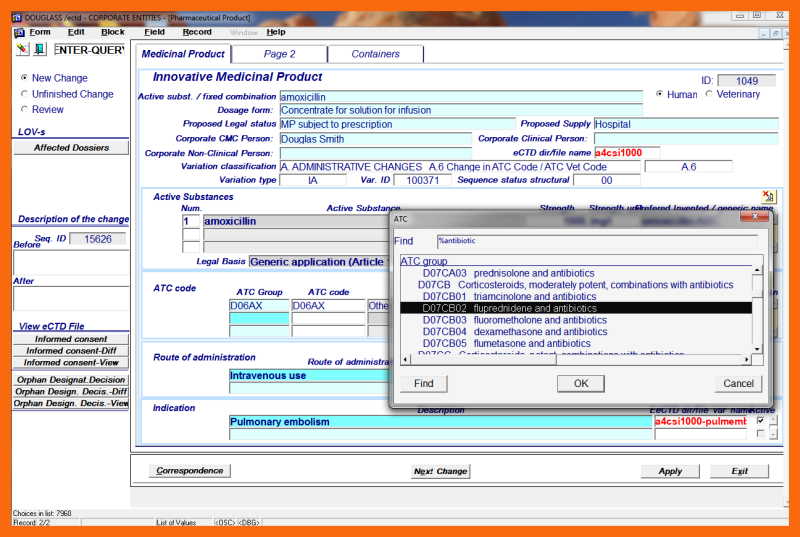
- A.6 Change in ATC Code/ATC Vet Code
- Variation related to the MP Safety, Efficacy and Pharmacovigilance Changes

Medicinal Product - structural change
For each Medicinal Product, a designated cmc entity person is named during the entity creation process.
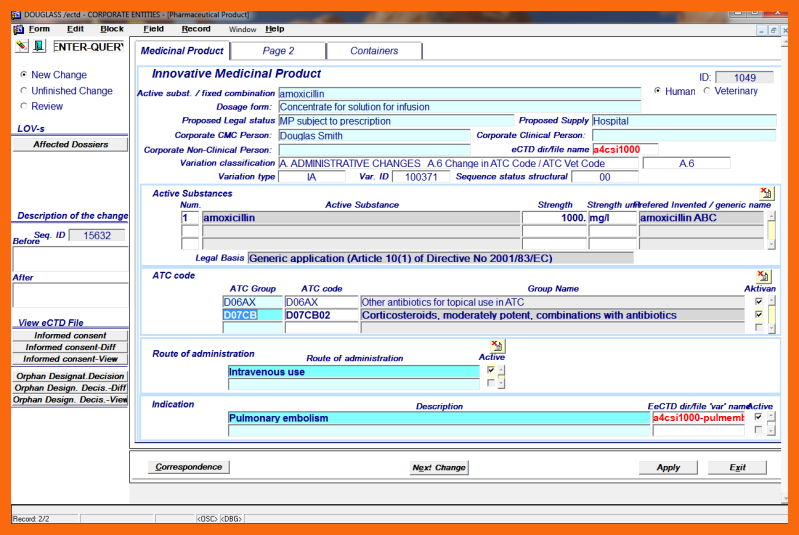
Only the designated cmc entity person, his Assistant, or his Agent using the application form "Corporate Entity Change / Medicinal Product / Structural Data" can introduce structural changes of the Medicinal Product into the eCTD Integrated Information System. Only one change can be introduced at a time.

Initiating structural change
A Corporate Entity can be changed only on the grounds of one or more "basis for change" emails having as attachments one or more "basis for change" documents defining the reasons for the upcoming change."
In the "New Change" mode of the application form using the LoV of Medicinal Products ready for change, with one or more unused "basic for change" emails, the selection of entity to be processed and structurally changed is performed. The logged-in person's roles as a designated follower, Assistant, and Agent are used as a filter to display a LoV of entities with upcoming changes.
After the selection of an entity that is subject to changes, using the "Affected Dossiers" button, a list of Dossiers that will be affected can be displayed.
On the same application form, in the mode "Review", it is also possible to review, with the person's clearance as a filter, all completely structurally defined Medicinal Products. In this case, changing the structural data is not possible.
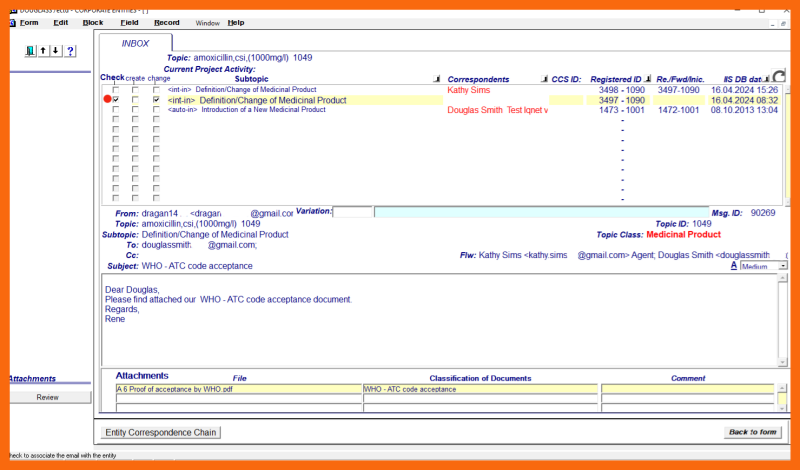
Using the dialog on the sub-application form "Correspondence" and received "basis for change" emails with attached documents, the designated cmc entity person his Assistant, or his Agent must determine the appropriate Variation type and start the structural definition of the change to the Medicinal Product entity.
Other non-"basis for change" emails related to the same Variation must also be identified and marked. From the LoV of possible Variations for the selected "basis for change" email combination, the appropriate Variation must be selected. If it is not certain which Variation is involved, additional "basis for change" emails with attachments must be collected to determine the appropriate Variation.
Using the "Back to form" button, a return to the main application form is made. When the change is applied and saved, a record of the selected Variation for a Medicinal Product change is inserted into the eCTD Integrated Information System. At the same time:
- a core sequence is generated and all subsequent actions of defining structural and narrative change are related to that core sequence
- initial submissions are generated in the affected Dossiers
- relevant regulatory project persons are informed via automatically generated enriched email notifications
- Corporate Projects are automatically launched in affected Dossiers and become topics in the Enriched Email
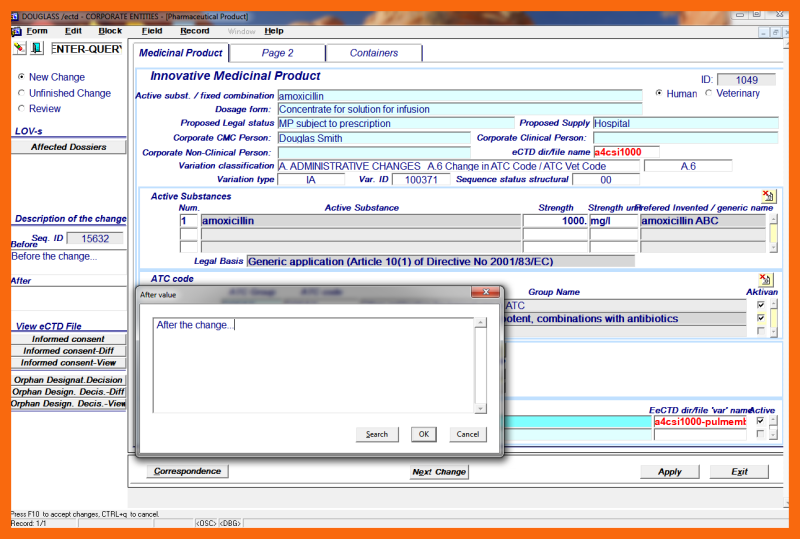
To finish the structural change short narrative "Description of the change" must be done using "Before" and "After" write boxes.

Completing the structural change
The structural change process can be completed in two ways:
- In one session when all changed structural data describing one Variation/change are defined
- in several sessions where
- in the first session appropriate Variation and some of the changed data are defined
- in the next session(s) when additional emails related to the Variation are marked and remaining additional changed data are defined
Additional sessions can be performed In the "Unfinished Change" mode of the application form using the LoV of Medicinal Product with selected appropriate Variation and unfinished change of data. The logged-in person's roles as a designated cmc person, Assistant, and Agent are used as a filter to display a LoV of entities with unfinished changes.
At the end of each session, the question "Is the structural change of the entity complete?" is automatically prompted and if the answer is positive, structural change is completed, the entity is removed from the LoV of entities with un unfinished change and assigned the status "completely structurally changed".
If the response is negative, the entity can be selected again using the option "Unfinished changes" when the LoV of entities with unfinished changes is displayed.

Medicinal Product - narrative change
At the insertion of a Medicinal Product change record into the eCTD Integrated Information System a directory tree structure of project initial submission documents and basic for change documents is automatically created.
For project initial submission documents, replacement files marked with (*) are created.
Replacement files for the already acquired basis for change documents are automatically substituted and marked with (#) and only the missing basis for change documents if any, are marked with (*).
Only the designated cmc entity person, his Assistant, or his Agent can change entity definition documents and upload them to the Corporate Documentation Cloud using the application form "Corporate Entity change / Medicinal Product / Narrative Data".
The designated cmc entity person his Assistant, or his Agent have to:
- Obtain all required basis for change documents from relevant Organizations
- Make necessary changes to the entity definition documents that have already been submitted to the Health Authorities and upload them to the Corporate Documentation Cloud
The local regulatory project persons have to:
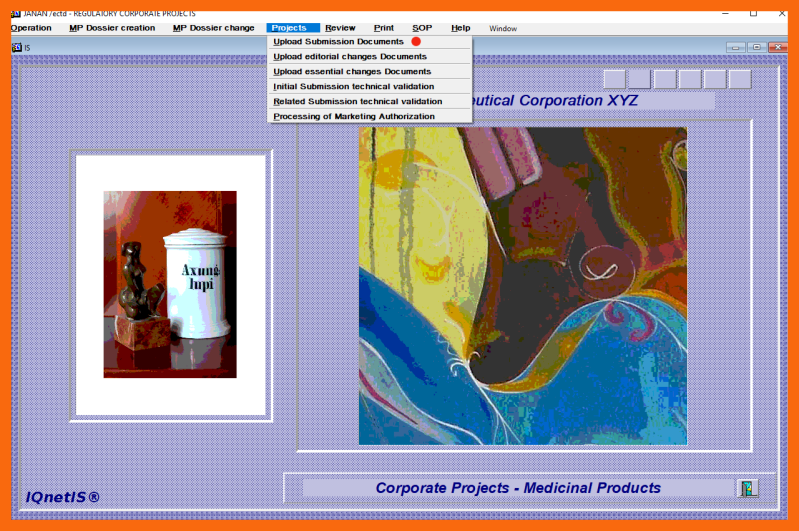
- Create project initial submission documents and upload them to the Corporate Documentation Cloud
Project submission documents are uploaded using the application form "Projects / Upload Submission Documents" from the "Corporate Projects - Medicinal Products" application module.
When the cmc entity person, his Assistant, or his Agent declares that the entity narrative change definition is complete, an automatically generated enriched email notification is sent to the regulatory project person so that the initial submission(s) can be submitted to the Health Authorities.